
How to draw Skeletal Formulae of Organic Molecules
Drawing Organic Molecules (in general)
There are several standard ways to represent the structures of organic molecules in different levels of detail.
These include:
- Molecular Formulae of Organic Molecules
- Structural Formulae of Organic Molecules:
- Fully Displayed Formulae
- Simpler Displayed Formulae
- Sketched 3D Formulae
- Modelled 3D Formulae
- Skeletal Formulae
For more about drawing organic molecules generally see: How to Draw Organic Molecules.
What are 'skeletal formulae' or 'skeletal structures' (of organic compounds) ?
In organic chemistry, skeletal formulae are the most abbreviated diagrammatic descriptions of molecules in common use. They look very bare because in skeletal formulae the hydrogen atoms (attached directly to carbons) are removed, leaving just a 'carbon skeleton' with functional groups attached to it.
Don't be fooled: The hydrogen atoms are present in the molecules but their presence is assumed, rather than drawn or stated, in the case of skeletal formulae.
This type of representation may take some getting used-to and is not always taught at school-level chemisty (so you may not need to know about use it, check your syllabus to be sure).
Simple Examples of Skeletal Formulae:
The simplest examples of skeletal formulae of organic molecules are those of linear alkanes.
Linear Alkanes: (Read more about alkanes)
Ethane |
||
 |
||
So, a 'chain link' of two carbon atoms linked by a single covalent bond (together with the hydrogen atoms attached to them) is represented by a single line when drawn in the form of a skeletal formula.
Further examples:
|
Propane |
||
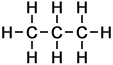 |
|
|
|
Butane |
||
 |
|
|
Pentane |
||
 |
|
|
Hexane |
||
 |
|
|
Heptane |
||
 |
|
|
Octane |
||
 |
|
|
Nonane |
||
 |
|
|
Decane |
||
 |
|
|
So, increasing lengths of alkane 'chains' of carbon atoms linked together by single covalent bonds (with the appropriate number of hydrogen atoms attached to each) are represented by a series of single short straight lines representing each 'link' in the 'chain', and these lines are arranged at the angles shown in the right-hand column (above).
Notice that it is not necessary to write the symbols 'C' or 'H' for atoms that form part of simple linear chains, as opposed to other structures such as functional groups attached to alkane-chains.
Examples of the Skeletal Formulae of a few Branched Alkanes:
Methylbutane |
||
 |
or any of the following equivalents:
|
|
Dimethylpropane |
||
 |
||
3,3-Dimethylpentane |
||
 |
|
|
Alkanes are the simplest examples of skeletal formulae because they involve only simple carbon chains and no other functional groups. See also more examples on the page about cycloalkanes.
Further examples:
Linear Amines: (Read more about naming amines)
Structure of Methanamine |
||
 |
 |
|
Structure of Ethanamine |
||
 |
 |
|
Structure of |
||
 |
 |
|
Structure of |
||
 |
 |
|
Structure of |
||
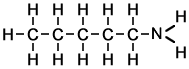 |
 |
The formulae of the first five linear amines (shown above) indicate how skeletal formulae are simplifications of the full displayed formulae of organic molecules.
One further set of examples is included below. These illustrate formulae of carboxylic acids, which are slightly more complicated than amines because carboxylic acids include a double covalent bond. Note how this is represented in the skeletal formulae by two parallel lines, just as it is also represented in the full displayed formulae by two parallel lines:
Linear Carboxylic Acids: (Read about naming carboxylic acids)
Structure of |
||
Structure of |
||
 |
||
Structure of |
||
 |
||
Structure of |
||
 |
||
Structure of |
||
 |
The above sets of examples are included because some people find it easier to understand skeletal formulae of organic molecules by looking at series of similar molecules (see also homologous series) and noticing trends through the series, as well as comparisons between drawings of the full displayed formula and the skeletal formula of individual molecules.
Why draw skeletal structures of organic molecules ?
Organic molecules can reach huge sizes. Sometimes it is necessary to describe the whole molecule but not to draw every single atom and chemical bond in full.
Organic molecules are often represented by skeletal formulae (i.e. only their skeletal structures) in cases of molecules beyond a certain size and complexity. Moderately advanced knowledge of organic chemistry is assumed.
The benefits of using the skeletal structures (which are sometimes referred to as 'skeletal formulae') include:
- Simpler (abbreviated) diagrams are generally quicker and easier to draw - by hand, or electronically.
- Simpler (abbreviated) diagrams often take-up less space.
- Important parts of the molecules are more obvious, i.e. have more prominence, in skeletal diagrams than in most fully displayed formulae.
- This type of diagram is commonly used in the chemical industry, hence useful to be familiar with.
When and where are skeletal formulae used ?
This type of representation of organic molecules is most frequently used in more advanced texts, research papers, and specialist areas. It is generally the most practical way to draw large and very complicated organic molecules. Even at lower levels of complexity (e.g. High School Chemistry and UK A-Level), skeletal formulae may be used - especially to describe structures involving carbon rings, such as cycohexane and benzene - see below.
Skeletal Formulae of Organic Molecules that have a 'ring', rather than a 'linear' or 'branched' structure:
Cyclohexane and Benzene
 |
 |
Structure of Cyclohexane
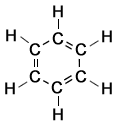 |
|
Structure of Benzene
Notice that the regular hexagons that form part (or all) of the skeletal formulae are approx. the same size as the carbon ring drawn out in the full displayed formulae on the left.
In the case of skeletal formulae, the presence of the appropriate number of hydrogen atoms attached to each carbon is assumed - UNLESS replaced by something else, e.g a carbon chain or functional group. For example, consider the structure and representations of ethylbenzene:
 |
 or  |
Structure of Ethylbenzene
As can be seen from the example of ethylbenzene (above), units of alkane chains are represented by straight lines at alternating angles, as indicated with examples further up this page.
More Examples of Skeletal Formulae
Note that some sources such as books, websites, reports etc., emphasize the presence of atoms other than carbon and hydrogen using colours, as in the following examples of skeletal formulae of predominately linear carbon-chain molecules. This representation (with use of colours) may be helpful but isn't strictly necessary and obviously cannot be used everywhere as some journals are printed in black ink only.
Propanoic Acid |
Hexanenitrile |
Propyl Pentanoate |
 |
 |
 |
Compare the above with the equivalent semi-displayed formulae (= 'simplified displayed formulae') below:
... and with the following fully displayed formulae:
 |
 |
 |
See also how to draw organic molecules.

 or
or 














