Naming Ketones
(Chemical) Definition of Ketones:
Ketones are organic chemical compounds that include a -carbonyl group (i.e. an oxygen atom attached to a carbon atom by a double covalent bond) such that the carbon atom to which the -carbonyl group is attached is itself attached to two other carbon atoms - as opposed to one other carbon atom and one hydrogen atom, which the case for aldehydes
That is, ketones are a class or category of organic chemical compounds that include a carbon atom attached to both an oxygen atom (by a double covalent bond), and also to two other carbon atoms (by a single covalent bond in each case).
Bearing in mind that carbon atoms form a total of 4 single covalent bonds - or equivalent in combinations of double or triple bonds, a carbon atom attached to both an oxygen atom (by a double covalent bond) and also to two other carbon atoms (by a single covalent bond in each case) cannot be the first- or last - (which are equivalent positions) carbon atom in the chain of carbon atoms that form the organic molecule of which it is a part.
This position of the -carbonyl group (oxygen atom) attached to a carbon atom that is not the last carbon atom in a carbon-chain is important because it distinguishes ketones from a similar category of organic compounds, called aldehydes.
In contrast to ketones, aldehydes include a -carbonyl group attached to the end-carbon in a carbon-chain.
Ketone molecules can vary in size up to very long molecules most of which consist of carbon atoms attached to each other and also to hydrogen atoms.
Names of Ketones in General
Ketones are named according to the same system as other organic compounds, with the suffix -one used to designate the presence in the molecule of a carbonyl group (that is, a carbon atom attached to an oxygen atom by via a double covalent bond) such that the carbon atom to which the oxygen atom is attached is not the last carbon atom in a chain or branch, but is always within (the middle of) a chain of carbon atoms. This may be easier to understand by looking at examples of the molecular structures of some simple ketones, as shown below:
As usual in organic chemistry, the first step to work out the name of a ketone molecule is the number of carbon atoms forming the longest straight, i.e. unbranched, chain within the molecule. Having identified the longest unbranched carbon chain within the molecule in terms of the number of carbon atoms in the chain, the main stem of the name of the chemical is as per the system used for naming alkanes.
If the carbon atoms do not form a linear chain but include branches, the longest linear chain of carbon atoms within the molecule determines the base of the name of the compound, onto which is added information about the branches incl. their lengths in terms of the number of carbon atoms in each branch (i.e. methyl- usually indicates a branch consisting of just one carbon atom attached to the main chain, ethyl- indicates a branch of two carbon atoms in length, etc.) and their positions along the longest linear carbon chain (e.g. attached to the 2nd carbon, 3rd carbon, etc.). Branched ketones are therefore more complicated to name than linear ketones.
The simplest linear ketones are named and their structures shown in the tables below.
Names and Structures of simple Linear Ketones
in which the -carbonyl group is attached to the 2nd carbon atom
It is useful to be aware that the same molecule can sometimes be drawn in several ways:
 |
2-butanone |
 |
 |
 |
|
The following table shows the names and structures of the first eight members of the homologous series of linear ketones in which a -carbonyl group is attached to the second carbon atom.
- 2-Propanone (C3H6O)
Simple Formula:CH3COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Butanone (C4H8O)
Simple Formula:CH3CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Pentanone (C5H10O)
Simple Formula:CH3CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Hexanone (C6H12O)
Simple Formula:CH3CH2CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
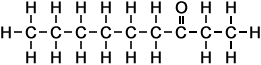
- 2-Heptanone (C7H14O)
Simple Formula:CH3CH2CH2CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Octanone (C8H16O)
Simple Formula:CH3CH2CH2CH2CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Nonanone (C9H18O)
Simple Formula:CH3CH2CH2CH2CH2CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 2-Decanone (C10H20O)
Simple Formula:CH3CH2CH2CH2CH2CH2CH2CH2COCH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names:
Names and Structures of simple Linear Ketones
in which the -carbonyl group is attached to the 3rd carbon atom
The first six members of the homologous series of linear ketones in which a -carbonyl group is attached to the third carbon atom is shown below.
- 3-Pentanone (C5H10O)
Simple Formula:CH3CH2COCH2CH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 3-Hexanone (C6H12O)
Simple Formula:CH3CH2CH2COCH2CH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 3-Heptanone (C7H14O)
Simple Formula:CH3CH2CH2CH2COCH2CH3Simple Structure:
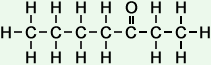 *Examples of other / previous names:
*Examples of other / previous names:
- 3-Octanone (C8H16O)
Simple Formula:CH3CH2CH2CH2CH2COCH2CH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 3-Nonanone (C9H18O)
Simple Formula:CH3CH2CH2CH2CH2CH2COCH2CH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: - 3-Decanone (C10H20O)
Simple Formula:CH3CH2CH2CH2CH2CH2CH2COCH2CH3Simple Structure:
 *Examples of other / previous names:
*Examples of other / previous names: